Product show
Dichloropropene (1,3-DCP)
Identification
Synonyms: (EZ)-1,3-dichloropropene (IUPAC); 1,3-dichloro-1-propene; 1,3-D; 1,3-dichloro-1-propylene; 1,3-dichloropropene; 1,3-dichloropropene (cis+trans); 1,3-dichloropropene (mixed isomers); 3-chloroallyl chloride; 3-chloropropenyl chloride; a-chloroallyl chloride; alpha,gamma-dichloropropylene; alpha-chloroallyl chloride; 'Telone II' (Dow AgroSciences). Other products 'Condor' (Dow AgroSciences); 'Curfew' (Dow AgroSciences); 'Dorlone' (Dow AgroSciences); 'Telone' (Dow AgroSciences); 'Telone EC' (Dow AgroSciences); 'Nematox' (Isagro); 'Rapsodi' (Hekta351); 'Trilone II' (Trical); mixtures 'Inline' (+ chloropicrin) (Dow AgroSciences); 'Telone C-17' (+ chloropicrin) (Dow AgroSciences); 'Telone C-35' (+ chloropicrin) (Dow AgroSciences); 'Agrocelhone' (+ chloropicrin) (AQL); 'Double Stopper' (+ chloropicrin) (Nippon Kayaku); 'Soilean' (+ chloropicrin) (Mitsui Chemicals); 'Telodrip' (+ chloropicrin) (DSBG); 'Telopic' (+ chloropicrin) (DSBG). Discontinued products 'D-D' * (Agro-Kanesho); 'Dedisol' * (La Littorale, Rhodiagri-Littorale); 'Nematrap' * (BASF); 'Sepisol' * (DuPont); 'Tellon 92' * (Sankei).
CAS No.: 542-75-6
EC No.: 208-826-5
Molecular Weight: 110.97
Molecular Formula: C3H4Cl2
Structural Formula: 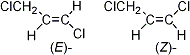
Form: Colourless-to-amber liquid, with a sweet penetrating odour (tech.).
M.p.: <–50 °C B.p. (E)- isomer 112.6 °C; (Z)- isomer 104.1 °C
V.p.: 3.7 kPa; (E)- isomer 2.3 kPa; (Z)- isomer 3.5 kPa (all at 20 °C)
F.p.: 25 °C (Abel closed cup)
Kow: logP = 1.82 (20 °C); (E)- isomer logP = 2.03; (Z)- isomer logP = 2.06 (both 25 °C)
S.g./density: 1.214 (20 °C); (E)- isomer 1.224; (Z)- isomer 1.217
Solubility: In water 2 g/l (20 °C); (E)- isomer 2.32, (Z)- isomer 2.18 (both in g/l, 25 °C). Miscible with hydrocarbons, halogenated solvents, esters and ketones.
Stability: Stable under normal conditions. DT50 11.3 d (pH 5–9, 20 °C).
Application
1,3-dichloropropene is used as soil fumigant nematicide in pre-planting control of most species of nematode in deciduous fruit and nuts, citrus fruit, berry fruit, vines, strawberries, hops, field crops, vegetables, tobacco, beet, pineapples, peanuts, ornamental and flower crops, tree nurseries, etc. Also has secondary insecticidal (soil insects) and fungicidal activity, and, by controlling nematode virus vectors, control is obtained of virus diseases of strawberries, raspberries, tomatoes, hops, etc. Applied at 60–350 lb/a. Phytotoxicity Can be phytotoxic if crops are planted too soon after application. Both isomers are active; the (Z)- isomer is the more effective to nematodes.
Specification
| 1,3-chloropropene (mixture of trans- and cis-) | 95.0% min. |
|---|---|
| specific gravity | 1.15~1.20 g/ml |
| boiling range | 115~126 deg. C |
| water content | 0.02% max. |
| Appearance | light yellow liquid |
Standard Packaging
230 kgs net drums, on pallet; 18400kg/20' FCL

Risks and Safety Information
1,3-Dichloropropene is Flammable, Toxic Liquid
Acute oral LD50 for rats 150 mg/kg. Skin and eye Acute percutaneous LD50 for rats 1200 mg/kg. Severely irritating to skin, eyes and mucous membranes. Prolonged contact with skin can cause severe burns. Inhalation LC50 (4 h) for rats 2.70–3.07 mg/l air. Birds LD50 for bobwhite quail 152 mg/kg. Dietary LC50 (5 d) for mallard ducks >10 000 mg/kg diet. Fish LC50 (96 h) for rainbow trout 3.9. Algae Slightly toxic. Bees LD50 (90 h) 6.6 μg/bee.


